Details of the Drug
General Information of Drug (ID: DM01HLX)
| Drug Name |
GR-127935
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
148672-13-3; GR 127935; GR-127935; N-(4-Methoxy-3-(4-methylpiperazin-1-yl)phenyl)-2'-methyl-4'-(5-methyl-1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-carboxamide; GR127935; UNII-2LLH6CEB40; 2'-Methyl-4'-(5-methyl-(1,2,4)-oxadiazol-3-yl)biphenyl-4-carboxylic acid (4-methoxy-3-(4-methylpiperazin-1-yl)phenyl)amide; 2LLH6CEB40; CHEMBL15928; GR 127935 hydrochloride hydrate; N-[4-METHOXY-3-(4-METHYLPIPERAZIN-1-YL)-PHENYL]-4-[2-METHYL-4-(5-METHYL-1,2,4-OXADIAZOL-3-YL)PHENYL]BENZAMIDE; CHEBI:64114; GR 127935 HCl; C29H31N5O3
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
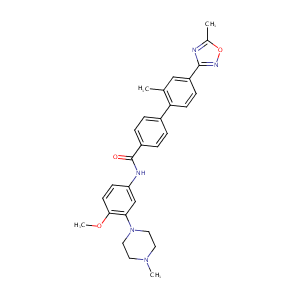 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 497.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
